MOLECULE
- Anti-bacterial and anti-fungal
- COX-2 inhibitor
- Free-radical scavenger
Regimen Lab Skincare Encyclopedia
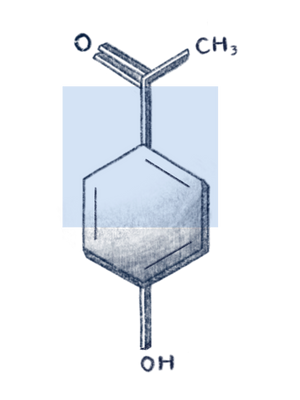
Hydroxy acetophenone
Regimen's Take
This is a fairly new multifunctional ingredient that acts as a preservative, antioxidant and soothing agent. It can’t kill microbes on its own but it is a good supporting agent if combined with other preservatives. Because it is a phenolic molecule, it is expected to have some antioxidant properties. It is worth adding to a formulation to lessen the irritation from traditional preservatives.
TLDR
- It is a multi-functional preservative
- It has soothing and anti-inflammatory ability
- It can support other antioxidants in the formulation
What is Hydroxyacetophenone?
Hydroxyacetophenone is a synthetic antioxidant and phenolic compound commonly used in cosmetics. It is naturally found in the Norwegian spruce (Picea abies) needles at low concentrations of 0.4-1.1%, dry weight. It can also be extracted from Cloudberries. Aside from its function as an antioxidant, it is reported to be a preservative (antimicrobial), skin-conditioning agent, as well as flavouring agent in non-cosmetic formulations.1 While the Cosmetic Ingredient Review recently published an extensive safety assessment on hydroxyacetophenone, only a limited number of clinical studies are available about the use of it as a major active component in cosmetics and therapeutics.
What is it used for?
Hydroxyacetophenone is a common ingredient in cosmetics for its antioxidant and soothing properties. It alleviates pain and irritation on the skin by inhibiting the COX-2 enzyme, which is responsible for the synthesis of prostaglandins during inflammation.2 The maximum concentration of hydroxyacetophenone is 5% in leave-on products with dermal exposure, such as face and neck products, while only at 0.23% in eye creams and eye makeup removers. Furthermore, it is used in soaps and detergents at up to 0.6%, and hairsprays and aerosol shaving creams at up to 0.5%.1 Reactivity or adverse effects from the use of commercial concentrations of hydroxyacetophenone in cosmetic products are not reported.
Biochemical Details of Hydroxyacetophenone
Role as an Antioxidant
Examples of stress our skin undergoes include UV exposure, sensitization, or injury. During these times, elevated levels of free radicals called reactive oxygen species (ROS) are generated. While our body naturally creates free radicals to maintain cellular integrity and immune function, persistently high levels of ROS during oxidative stress can cause DNA damage, protein carboxylation, and lipid peroxidation — all of which trigger a range of chronic health problems, including premature aging and cancer.3,4 Hence, antioxidants and anti-stress agents in cosmetic formulations play an important role to neutralize excess free radicals.
Phenolic compounds act as antioxidants in a number of ways due to its hydrogen-donating hydroxyl group. When a phenolic antioxidant interacts with a free radical during its scavenger hunt a hydrogen is transferred from the antioxidant to the free radical, and a radical form of the antioxidant is generated. This form of the antioxidant has much greater stability than the initial radical due to delocalization of its benzene ring. The reaction ultimately neutralizes the initial radical and terminates the cycle of generating new ones.4,5 Hydroxyacetophenone shows dose-dependent antioxidant ability and works synergistically with other antioxidants to enhance anti-oxidative capacity.

Anti-inflammatory property
The anti-inflammatory activity of hydroxyacetophenone underlies some of the key mechanisms behind its function as a skin-conditioning agent and anti-irritant. Cellular signaling during an inflammatory response upregulates the expression of the COX-2 enzyme, which is the most important enzyme in the synthesis of prostaglandins. These are lipids found at high concentrations in an injured or infected site to modulate the inflammatory response, and contribute to the perception of pain by sensitization of primary nociceptive nerve endings.6 Inhibition of the COX-2 enzyme and consequently prostaglandin production using non-steroidal anti-inflammatory drugs (NSAIDs) or other agents with anti-inflammatory properties, such as hydroxyacetophenone, can both alleviate the effects of inflammation and prevent further damage of the affected cells. Hydroxyacetophenone shows to have some COX-2 inhibitory property compared with Resveratrol as positive control.

Does it penetrate the skin?
Data on skin penetration is not available. Studies focusing on hydroxyacetophenone as a major active ingredient in cosmetics are very limited at the moment.
Safety Assessment
| Concentration of Sample | Study type | Results/ Conclusion |
| 0.5g pure Hydroxyacetophenone moistened with Saline applied to clipped skin and occluded after application | In-vivo. White rabbits :( n=4 | Slight dermal irritation was reported for 3 of the 4 test animals, including minimal erythema, without edema. 7 (Note: they used pure Hydroxyacetophenone) |
| 0.5g pure Hydroxyacetophenone moistened with Saline applied to clipped skin and occluded after application |
In-vivo. White rabbits :( n=6 |
All control and treated sites were free of dermal irritation throughout the study period. 8 |
| 3%, 5%, 15%, and 30%, respectively, in 4 different vehicles: tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), methanol (MeOH), or N,N-dimethylformamide (DMF). |
In-vivo. White rabbits :( n=24 |
The test article did not significantly increase the dermal irritancy of any vehicle. 8 |
| 0.5% Hydroxy-acetophenone in propylene glycol (intradermal injection, then topical), 0.5 g of 75% hydroxy-acetophenone in petrolatum (topical challenge) | In-vivo. Guinea pigs :( n=20 | No sensitizing reactions were observed.8 (Note: One Case Study showed that sensitization is possible in humans)9 |
References
1. Safety Assessment of Hydroxyacetophenone as Used in Cosmetics. (2021). Cosmetic Ingredient Review. https://cir safety.org/sites/default/files/hydace032021SLR.pdf
2. Chang CW, Chen YC, Lin YC, Peng WH. (2017). p-Hydroxyacetophenone suppresses nuclear factor-κB-related inflammation in nociceptive and inflammatory animal models. J Nat Med. 71(2): 422-432. doi: 10.1007/s11418-017-1074-9
3. Kinjo Y, Takahashi M, Hirose N, Mizu M, Hou DX, Wada K. Anti-stress and Antioxidant Effects of Non-Centrifuged Cane Sugar, Kokuto, in Restraint-Stressed Mice. Journal of Oleo Science. 68(2): 183-191. doi: 10.5650/jos.ess18198
4. McMullen RL. (2019). Antioxidants and the Skin (Second Edition). CRC Press
5. Pereira DM, Valentão P, Pereira JA, Andrade PB. Phenolics: From Chemistry to Biology. Molecules. 14, 2022-2111. doi: 10.3390/molecules14062202
6. Ricciotti E, FitzGerald GA. (2012). Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 31(5):986-1000. doi: 10.1161/ATVBAHA.100.207449
7. Hoechst Celanese Corp. Acute Skin and Eye Irritation in Rabbits. U.S. Environmental Protection Agency (EPA);1985.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/OTS0570600.xhtml. Accessed January 29, 2021.
8. European Chemical Agency (ECHA). REACH registration dossier: 4'-hydroxyacetophenone (CAS 99-93-4).
https://echa.europa.eu/registration-dossier/-/registered-dossier/11354/1. Last Updated: 2020. Accessed:
02/10/2021.
9. Sanz-Sánchez T, Garrido R, Cid P, Díaz-Díaz R. Allergic contact dermatitis caused by hydroxyacetophenone in a face
cream: Allergic face dermatitis caused by Hydroxyacetophenone. Contact Dermatitis 2018;78:174-175.
